All the elementary particles and the composite particles they make up have wave-particle duality. The universe is best understood as localized vibrations in quantum fields and the interactions of those fields. Those excited states have the properties of a particle and a wave, thus wave-particle duality is a very fundamental aspect of the universe.
All Elementary Particles Exhibit Wave-Particle Duality fact
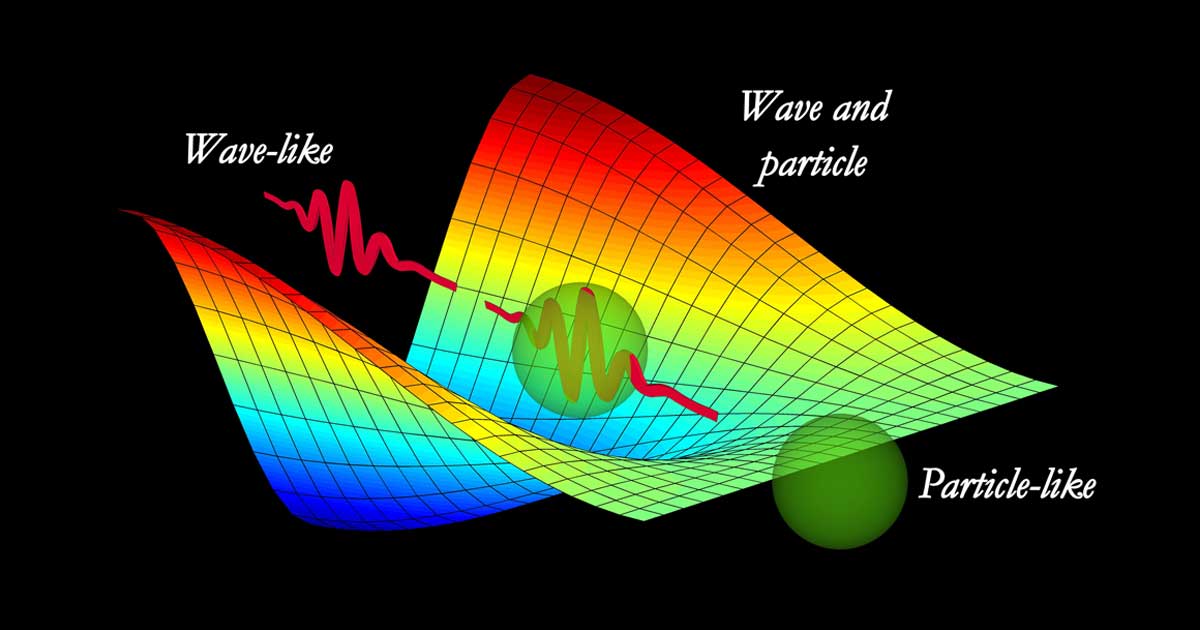
Are All Quanta Both a Particle and a Wave?
You may have heard “light” (the photon) is both a particle and a wave, and this is true, but the photon isn’t the only elementary particle that exhibits wave-particle duality.
All elementary particles exhibit wave-particle duality, acting as both a particle and a wave.
All elementary particles are localized vibrations in their respective quantum fields, which propagate in a wavelike manner (waves). Each particle’s wave-like and particle-like qualities are two complementarity observable aspects of a single phenomenon that can be described in terms of excitations in a quantum field.[1] This concept is explained by Quantum Field Theory (QFT).[2]
A single particle is usually comprised of a “wave packet” composed of many waves creating one aggregate wavelength. Each one can radiate bosonic energy, which is typically photons (light). In theory, each single wave in the wave packet can, in turn, be thought of as a one-dimensional vibrating “string” (Super String Theory), although their field-like and wave-particle nature is better tested.
The following video explains this in simple terms.
TIP: If the quantum nature of particles feels a little heady, consider checking out a primer on particle physics for kids.
Quantum Field Theory by Fermilab. The Fermi National Accelerator Laboratory creates some excellent videos. This is a very simple explanation of quantum field theory, which explains the particle-wave-field nature of quanta.Quantum Field Theory (QFT) and Wave-Particle Duality
Quantum Field Theory is a theory of quantum physics that describes all quantum particles (quanta) as localized vibrations in a quantum field. Each type of particle has a single field attributed to it that spans space. Any vibration in that field (a measurable excited state of any frequency) is a particle.
That particle travels in a single direction, at an infinite range, in a wave-like manner, in a state of unpredictability (quantum superposition). Despite its unpredictable nature, all quantum particles quantize to discrete, measurable states in space and time that can be measured as both wave-like and particle-like. The faster the vibration, the easier it is to measure in space as the distance between crests occurs over less time. One way to increase or decrease the frequency of vibration is if the particle is affected by something (i.e. another particle, or lack of other particles).
When two charged fields overlap, we get the particle interactions as explained by the standard model. Thus composite particles, like the proton or even atom, can be described as overlapping excited states of quantum fields, as can the things they make up like molecules.
To clarify:
- Quanta act like waves because they travel as transverse waves (a wave vibrating at right angles to the direction of its propagation) within their respective fields.
- Quanta act like particles because they “quantize” (ball up) into discrete excited energy states within their respective fields.
With the above in mind, the classical concepts of “particle” and “wave” don’t fully describe the behavior of quantum-scale objects. Thus, despite working theories, there is no single way to describe what it means to have wave-particle duality. Quantum field theory is the best current model for understanding the essence of quantum particles.
FACT: Fields overlap, but two excited energy particles with mass, and the same charge can never occupy the same space (instead particles repel “like” charges and attract opposite charges).
TIP: Whether we measure quantum particles as waves or particles largely depends on what we are doing. They can be measured either way due to their dualistic nature.
TIP: The shorter the crests of a wave, the easier that wave is to measure as a point in space, the higher the energy content of the particle, and the “hotter” that particle is (more energy = “hotter”).
Understanding Particle Wave Duality With Examples
To help internalize how quanta can be both a particle and a wave, it may help to think about the familiar electron as an analogy.
Consider an electron cloud orbiting an atom (both electrons and light are essentially electromagnetic energy, one of four fundamental forces). The electron exists in a state of probability, propagating as a transverse wave, with measurable excited states called electrons, orbiting an atom so fast it creates “a cloud of potential and actual locations” in the electromagnetic field.
Despite its field-like and wave-like nature, the electron is still measurable as a single excited state at any point.
Orbitals: Crash Course Chemistry #25. This video explains orbitals which should help you get a visual of what it means to be a particle and a wave.TIP: A classical “wave” has a continuous value, but in the quantum world things quantize to discrete values. Where particles will appear is governed by “uncertainty” and probability, but based on the constant nature of light (Planck constant and light speed).
TIP: Key concepts show that everything is, at its core, is mass-energy field interactions whose energy can be conserved in other forms, but never created or destroyed. These concepts include mass-energy equivalence (which deals with the fundamental properties of quantum particles); the uncertainty principle; superposition; the laws of conservation; and universal constants like the Planck length and light speed.
Quantum Theory – Full Documentary HD.