Mass and energy can’t be created or destroyed. At it’s core all that exists has always existed and always will exist.
Energy is Neither Created or Destroyed fact
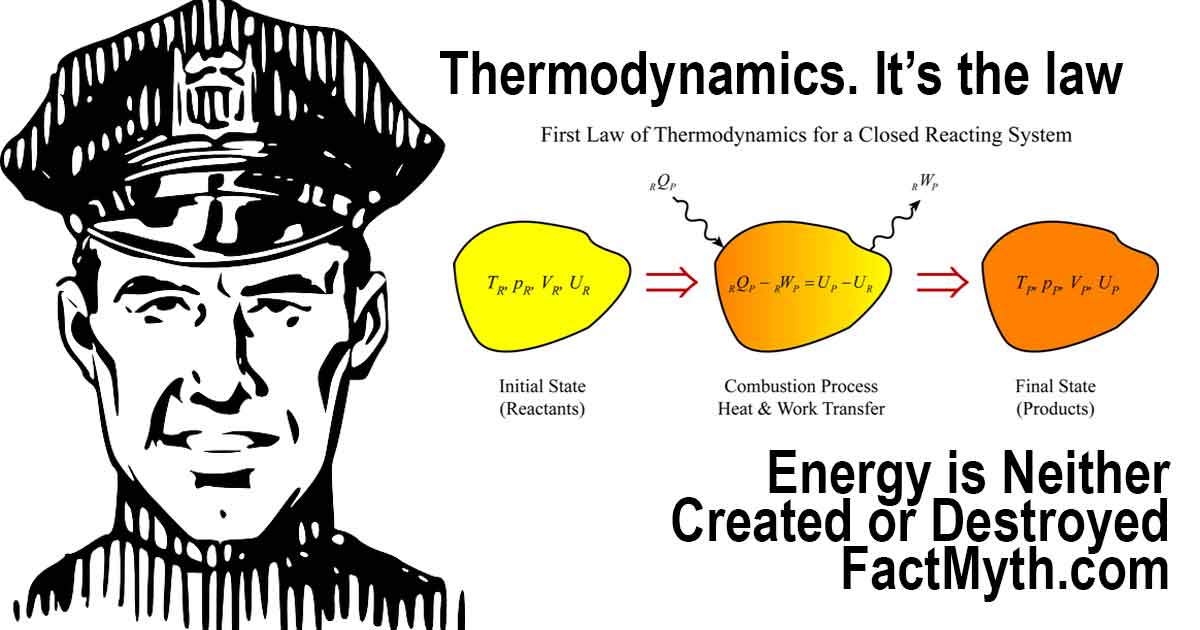
Can Energy Be Created or Destroyed?
Neither energy nor mass can be created or destroyed. Although energy can change forms, all energy in a closed system and must remain constant.
- The law of Conservation of Mass: Mass can not be created or destroyed.
- The law of Conservation of Energy: Energy can not be created or destroyed.
- The law of Conservation of Mass-Energy. Neither energy nor mass can be created or destroyed, instead mass is conserved as energy or energy is conserved as mass. A combination of the law of conservation of energy and conservation of mass (based on E = mc2 (energy mass equivalence)).
Can Mass-Energy Be Converted?
Despite it’s common use, the phrase “converted into” is a point of contention among scientists. The correct phrasing is “conserved as”. The building blocks of the universe don’t change. Mass isn’t converted into energy, or vice-versa, they are “conserved” as each other. The correct way to phrase the underlying core principle of conservation is “mass-energy is conserved”. There will never be any more or less mass-energy in the universe than there is right now, but it changes forms constantly.
The Laws of Conservation (AKA Universal Conservation Laws)
Mass-energy isn’t the only thing that can’t be created or destroyed. Essentially, none of the fundamental properties of particles can’t be created or destroyed including (mass-energy, spin, momentum, and charge).
In overly-simple terms, all mass-energy arises from the same fundamental forces, thus it is those forces which are changing forms, but never created or destroyed. Understanding the conservation of mass-energy is the key to it all, so let’s stick with that below. Read more about conservation laws here and here.
TIP: Even probability is conserved in the physical universe. Generally, if nothing else, everything is conserved as “pure massless energy” AKA the photon (such is in the case of an elementary anti-particle colliding with a particle and annihilating itself).
What is the Law of Conservation of Mass?
The law of conservation of mass says, “in a chemical reaction, mass is neither created nor destroyed”.
What is the Law of Conservation of Energy?
The law of conservation of energy, also known as the first law of thermodynamics, says: “all energy in a closed system must remain constant, it can neither increase or decrease without interference from the outside”. The law of conservation of energy has shown to be absolute.[1]
What is the Law of Conservation of Mass-Energy?
The law of conservation of mass-energy says mass and energy have equivalent value and can be conserved as each other. This doesn’t mean they are exactly the same on all levels, but rather they are simply equivalent and follow the same laws.
We can combine mass conservation and energy conservation into one neat little package of mass-energy conservation. E = mc2 (Einstein’s energy mass equivalence).
A video discussing E = mc2 and the difference between mass and energy.What is Matter? (Mass–Energy Equivalence)
Matter is energy and mass (specifically fermion and boson interactions). Everything in the universe has the measurable property mass-energy. Everything from gravity, to electricity, to the stars, to you and the chair you are sitting on is simply mass-energy taking different forms.
We know that mass and energy are conserved as each other because of Albert Einstein’s famous E = mc2 equation which shows “massless energy particles can act as mass when bound to a system“. Energy = Mass times the constant speed of light squared.
A more technical look at E = mc2 , how mass and energy relate to each other.Can Matter Be Created or Destroyed?
Matter can be destroyed and created, but its building blocks of energy and mass can’t. Instead energy and mass are simply conserved as each other when matter transforms.
The Difference Between Matter, Mass, and Energy
All matter is essentially condensed energy and all energy “has mass” when bound to a system.
Even though they are equivalent and able to “convert” into each other without losing or gaining total mass-energy, energy and mass aren’t exactly the “same”. You can think about it like this, energy is the fundamental building block of everything in the universe and all energy “has mass” when bound to a system.
That said, this is semi-laymen speak, science differentiates between mass and energy in a number of complex and subtle ways. Given the technical aspect you should not try to divide mass and energy, rather you should be like Einstein and simply say “mass-energy” to talk about both properties as a whole.
What is the First Law of Thermodynamics?

The First Law of Thermodynamics illustration.
The key to understanding that energy is neither created nor destroyed is understanding the first law of thermodynamics (the law of conservation of energy).
In laymen’s terms, the total energy in the system is not affected by energy lost by the system due to work or energy added to the system due to a heating process. Regardless of what happens in the closed system all energy remains constant.
In more technical terms, for a closed thermodynamic system, the first law of thermodynamics may be stated as:[2]
 , Or equivalently,
, Or equivalently, 
 is the amount of energy added to the system by a heating process,
is the amount of energy added to the system by a heating process, 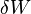 is the amount of energy lost by the system due to work done by the system on its surroundings and
is the amount of energy lost by the system due to work done by the system on its surroundings and 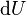 is the change in the internal energy of the system.
is the change in the internal energy of the system.
How Does the Law of Conservation Apply to Our Universe?
All matter has a measurable property of mass and energy. Energy can be “converted” into mass, and mass can be “converted” into energy, but the total sum of mass-energy in the universe will never change.
The energy in our universe has always existed and will always exist. It simply changes forms. At any moment the energy in our universe will, theoretically, always be measured as being exactly the same.
But I Heard They Destroyed or Created Energy?
Even when we seem to “create something from nothing” or “turn something into nothing” this isn’t what is actually happening. Energy can appear not to be conserved in quantum fluctuations for small amounts of time while particle-antiparticle pairs are created. However, as far as we know at this point in time energy is always conserved. In fact, there is no such thing as “nothing”. The universe is full of “somethings” like quantum field fluctuations.
Is the Universe a Closed System?
The universe can be described as an open, closed, or isolated system depending upon how we look at it although, this doesn’t affect the law of conservation of energy.
There are 3 main types of thermodynamic systems, defined by what the system can exchange with its surroundings:[3]
- An open system can exchange both energy and mass.
- A closed system can exchange only energy.
- An isolated system cannot exchange anything.
The entire universe (known and unknown) is an isolated system, in simple terms a universe that comprises everything and can’t exchange energy with anything, as nothing else exists. The observable universe is an open system that can exchange energy. Potentially, the multiverse could lead us to rethink how this works.
The fact that the first law of thermodynamics uses a closed system to prove energy conservation doesn’t imply that energy can be destroyed or created in an open or isolated system.
How Does Energy Change Forms?
Energy can change forms in a number of ways. Consider first that everything in the known universe is comprised of energy in different forms.
Example: When you climb stairs, chemical energy in your food is changed into kinetic energy by your muscles, and into potential energy as you raise your body against gravity.[4]
But Does it Fall Apart at a Quantum Level?
Quantum mechanics (how physics works in the micro-verse) poses some strange problems for energy conservation, but it has not yet presented any unsolvable problems. Quantum physics says that electrons like to “quantize”. In other words, electrons jump from point A to point B (instead of moving from point A to point B in a straight line).[1]
Not even quantizing packets of energy can defy energy conservation. Although an electron will fluctuate at a quantum level (being more than one place at once and seemingly not conserving energy) the total energy of the electron and its radiation remain constant.
But Space is Expanding, So Where Is That Extra Matter Coming From?
Space is thought to be expanding faster and faster propelled by “dark energy” (intrinsic energy at the center of the universe). We do not know how dark energy can spawn empty space, which must contain more intrinsic energy, without creating additional energy? Einstein believed that the answer is to be found in general relativity.[1]
Regions of space with positive energy actually push space outward. As space expands, it releases stored up gravitational potential energy, which converts into the intrinsic energy that fills the newly created volume.[1]
Does This Mean My Body is Made Up of Energy that Has Been and Always Will Be?
We are all made of energy that has always and will always exist.
- “Fact or Fiction?: Energy Can Neither Be Created Nor Destroyed“. Scientificamerican.com. Retrieved Jan 14, 2016.
- “Conservation of energy“. Wikipedia.org. Retrieved Jan 14, 2016.
- “Is the universe an open or a closed system?“. quora.com. Retrieved Jan 14, 2016.
- “ENERGY“. Infoplease.com. Retrieved Jan 14, 2016.
- “The Conservation of Mass-Energy“. Chemteam.info. Retrieved Jan 14, 2016.

 , Or equivalently,
, Or equivalently, 
Gareth Morgan Did not vote.
In a closed system at room temperature comprising a tank containing 1 liter of air at 10 bars of pressure which is released to flow into a second, evacuated tank of 9 liters, the temperature of the tanks and the air will fall to below freezing. Heat energy is lost as well as the potential energy in the compressed air and the vacuum. Those forms of energy have been “destroyed” in carrying out the work of expanding the compressed air. There is no increase in kinetic, potential, heat, electromagnetic or any other form of energy.
Einstein’s statement that energy cannot be destroyed appears to be mistaken.
Thomas DeMicheleThe Author Did not vote.
Interesting, “something” must be happening in this experiment.
I would be very happy to add the argument to the page, but we need a citation. Can anyone find the study that shows this?
Here is an article that comes close to discussing this: https://www.physicsforums.com/threads/what-happens-to-energy-as-air-fills-a-vacuum.700152/
Charles Did not vote.
Myth
Thomas DeMicheleThe Author Did not vote.
How do you think, say mass-energy, can be created or destroyed and not simply conserved in another form?