The Bohr model has been outdated since Schrödinger’s equation and the discovery of quantum mechanics. The structure of the atom is now a completely solved problem – so much so that the same model can be applied to describe the behaviour of molecules, rather than just single atoms, completely, as well as the behaviour of solid materials. But alas, the simple 2D solar system Bohr model will likely remain with us for a long time as a starter kit due to its simplicity, recognizability, and importantly it being easy to relate to our solar system. For much the same reason we teach F=ma gravity (and not warped spacetime), we teach the sometimes useful and always simple Bohr model.
The Bohr Model is the Most Accurate Model of an Atom myth
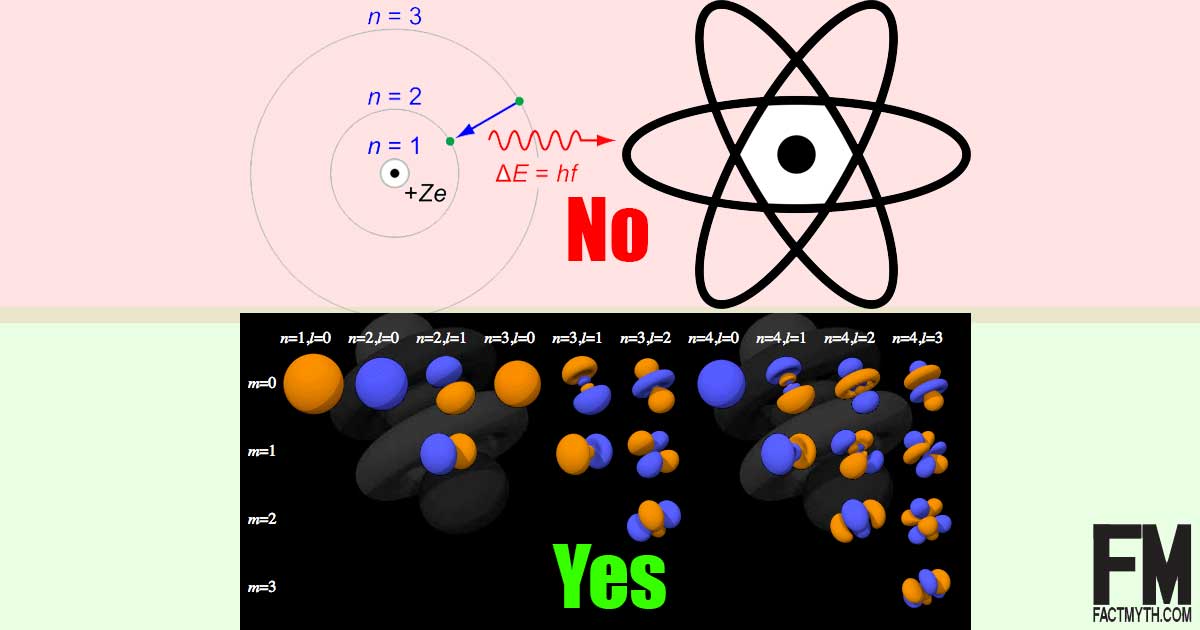
Is the Bohr Model of the Atom Accurate? Is the Quantum Model of the Atom More Accurate than the Bohr Model?
The Bohr model of the atom (1913), the one that looks like a solar system, has been replaced by the more accurate Quantum Model of the Atom since 1927.[1][2][3][4][5]
In other words, Schrodinger’s model of the atom (the quantum model that shows “electron clouds“) is the most accurate representation of an atom. With that said, Schrodinger built on the work of Rutherford, Bohr, and Sommerfeld (hovering on the shoulder’s of giants at about 10^-8 meters).
Models of the Atom Timeline. This article works well to start because it presents a timeline of atom models right off the bat. Here we see the Thompson (bread pudding) and Rutherford (nuclear) models that lead to the Bohr model, which was extended by Sommerfeld, and finally replaced by Schrödinger’s Quantum Mechanical Model.FACT: Niels Bohr’s “Bohr model” is also known as the Rutherford–Bohr model, as the Bohr model built on Ernest Rutherford’s model which proposed that electrons orbited the nucleus much like a planet around the Sun. This was later improved and extended to become the Rutherford-Bohr-Sommerfeld model by Arnold Sommerfeld. The Quantum Mechanical Model is also known as Schrödinger’s model (the same Schrödinger as Schrödinger’s cat) as Schrödinger applied new quantum theory to Bohr’s model (Werner Heisenberg also helped to create Schrödinger’s electron cloud model).
The Electron: Crash Course Chemistry #5. This video will help explain the concept of “electron clouds.”Why Isn’t the Bohr Model Accurate, Why Use Schrödinger’s Model?
It isn’t that Bohr’s model is completely inaccurate, it is that its 2D depiction of the atom is misleading, leaves out some key factors, and doesn’t work with heavier elements.
The quantum model (Schrödinger’s Model) describes the wave-like properties of the quantum particles that make up atoms better, specifically the behavior and properties of electrons orbiting the atom. However, quantum theory was developed after Bohr presented his solar system-like model.
In real atoms, electrons aren’t tiny stationary dots in planet-like orbit around the nucleus. Rather, electrons surrounding an atom exist in a state of probability (quantum superposition) as “electron clouds.” They move at fractions of light speed (about 1% of light speed),[6] they move in many different directions, and their location can be predicted only as a probability and not with exactness.
FACT: Theoretically an electron can be nearly infinite distance away from the atomic nucleus it is orbiting. With that said, the probability of finding an electron decreases dramatically the further away from the nucleus you search. The places the electron is most likely to orbit is represented by the “atomic orbitals” illustrated in the electron cloud model.[7]
TIP: Atoms exchange electrons constantly via “covalent bonds.” So electrons are moving through systems of molecules at speeds of up to 1% of light speed in all directions, always, as they are shared between atoms in a physical system.
Quantum Mechanical Model. Paul Anderson’s series Bozeman Science deserves a nod. He tackles difficult physics and chemistry concepts in a simple way. I highly recommend this video that compares the quantum model to the Bohr model, as well as his other videos.TIP: Wolfgang Pauli, another great thinker and scientist of the era, contributed to atomic and quantum theory by stating that two identical things couldn’t be in the same place at the same time(Pauli exclusion). His theories help explain how negatively and positively charged electrons orbit an atom in orbitals.
Orbitals: Crash Course Chemistry #25. This video will help explain the concept of “electron orbitals” (not “orbits”).
The famous 1s, 2s, 3s, 3p … orbitals of a hydrogen atom (a quantum model of a hydrogen atom), showing electron clouds.
How Does Bohr’s Model of the Atom Differ From the Quantum Mechanical Model?
The models differ in a few ways, but importantly:[8]
- The quantum model represents the true 3D space an atom exists in, the Bohr model only represents a 2D space.
- The Bohr model was a 1D model that used one quantum number to describe the distribution of electrons in the atom. The only information that was important was the size of the orbit, which was described by “n” the principle quantum number.
- The Bohr Model treats the electron as a particle in fixed orbits around the nucleus. The Quantum Mechanical Model treats the electron mathematically as a wave. The electron (like all elementary quantum particles) has properties of both particles and waves.
Why Do We Teach Children an Inaccurate Model of the Atom?
The Bohr model has long been considered obsolete by chemists and physicists, yet it is still taught purposefully in classrooms today due to its simplicity.
The Bohr model helps transmit the basics of atoms to new students. Unfortunately, it causes confusion down the road when the student learns that electrons are wave-like clouds of electrons moving at fractions of light speed in a state of probability following the rules of quantum mechanics, and not tiny dots surrounding a nucleus like the planets around the sun.
The History of Atomic Chemistry: Crash Course Chemistry #37. CrashCourse is an amazing series. Here is the history of atomic history.Two Justifications for Teaching Bohr’s Model
Below are two key reasons to teach the inaccurate Bohr model:
- It reminds students how theories work. Science often revolves around building models (AKA theories), and it is very common that a new better model comes along to replace the older model. This rarely makes the old model completely wrong. As a novice might expect, we all need to grasp this fundamental aspect of science. (See our page on “how theories work“).
- The Bohr model is simple. It is a 2D model that correctly explains many aspects of the Hydrogen atom, and that is a good starting point for learning about atoms. The Quantum Model represents 3D objects moving in quantum superposition. It gets rid of little dots and replaces them with clouds of probability moving at speeds of a mean speed of 1/137th the speed of light (for electron clouds in a hydrogen atom). The Quantum Model factors in sophisticated concepts: principal quantum number: n; Angular momentum quantum number: l (with a different letter for value’s of l in sub-shells like s,p,d,f,g); Magnetic quantum number: m1; Spin quantum number: m2. When we realize the complexity of the Quantum Model, we can see why teaching the Bohr model can be attractive.
Take one look at a simple explainer for the quantum model of an atom and you’ll immediately see that while teachers may mention the quantum model, most 101 level classes will continue to teach the simpler 2D Bohr model despite its long-known inaccuracy.[9]
History of the Atom. This is the AP version of the history of the atom.- The development of the atomic model
- Atomic Structure: The Quantum Mechanical Model
- What is the currently accepted model of atomic structure?
- Erwin Schrödinger
- Niels Bohr
- How fast do electrons move?
- Electron cloud
- https://socratic.org/questions/how-does-the-bohr-s-model-of-the-atom-differ-from-the-quantum-mechanical-model
- Why It’s Okay to Teach Wrong Ideas in Physics
Check out this Quora post for a simple and true breakdown of “What is the currently accepted model of atomic structure?” It walks through the old models and talks about how we arrived at better ones. It is a bit heady, but worth the read.
Also, check out “A New Model of the Atom” which is one person’s attempt at updating the Quantum Model of the atom. Also from Wikibooks, this simple walkthrough of the History of the Atom is worth a read.

real studemt Supports this as a Fact.
FAVCTAY GOLIDSVBGULIASDHBOGI;ASDF;IHOABOGMER;OHIAERJHPOJBGOI;SJOIFAAAAAAAAAAACT FACT
thushara Did not vote.
rutherford’s model is called as planetary model.what he said is wrong . bohr proposed elliptical orbits.
Shay Did not vote.
Thnks