What is Light?
Understanding the Nature of Light (And Thus Photons and Electromagnetic Energy)
We explain “light,” both as electromagnetic radiation within a visible portion of the electromagnetic spectrum, and as electromagnetic energy carried by photons.
In other words, on this page we are using light as a placeholder for all things electromagnetic from the photon, to the virtual photon, to electromagnetic energy, to electromagnetic radiation, etc. That is, the photon in any form and understood any way.[1]
TIP: Below we define “light” according to quantum field theory (where it is a particle-wave vibrating in the electromagnetic field), the standard model (where it is, in simple terms, the force carrier for the electromagnetic force, the photon; with this being true even when static via virtual photons), and the Copenhagen interpretation (where it is a particle-wave in quantum superposition). That is, we are defining light as a photon and its effects, through the lens of different models. These aren’t the only theories (models) for understanding light/photons/electromagnetism or quantum physics in general, but these are widely accepted theories that work well to introduce the fundamental property of the universe we here are calling “light.”
What is Light?
Light, understood broadly as all electromagnetic energy, is a core property of the universe. As such it is not so easily explained.
When we say light in common language, we simply mean the visible portion of the electromagnetic spectrum that our eyes can detect and process as visuals.
However, if we understand light more broadly, we can say any portion of the spectrum, visible or not, is “light.” This means radio waves, wi-fi, radiation, heat, all of it, is all “light.”
When we take into account that photons are the carrier particle for electromagnetic energy, we can say: photons (the carrier of electromagnetic energy), electromagnetic force (one of four fundamental forces in the universe), electromagnetic radiation or energy in any form, and in simple terms “light” are all different words describing the same fundamental phenomena.
With that in mind, we can offer a more complex definition of light as a wave-particle in a quantum field where we can say: “Light” is a particle (photon), that acts like a wave, and is understood as a localized vibration in the electromagnetic field. Or, we can describe light as a wave function of probable locations of excited states in the electromagnetic field in superposition that can be measured as “particle-like or a wave-like” and travel in spacetime as electric and magnetic polarized transverse waves (unless they are behaving as virtual photons) moving in a straight line at a constant speed when unimpeded but manifesting as localized vibrating wave-packets in the electromagnetic field (something like that).[2]
All the above definitions of light are essentially correct, even if each only hints at the true dualistic and quantum nature of that electro-magnetic force that exists as a wave-particle that is so closely related to mass-energy.
From here we could go into what is almost a poem about light (which remember we are considering as a synonym for electromagnetic energy and the photon), where we describe many aspects of it rather than trying to give it a single definition:
- Light has a dualistic particle-wave like nature (as do all “quanta” in the standard model).
- Light is pure electromagnetic energy (and is also thus the carrier particle the “photon”).
- Light is one of four forces (AKA quantum interactions) in the physical universe.
- Light exists as a charged energy field, and it exists in a quantum state (it is a “quantum particle,” with quantum behavior, just like the other quanta studied in quantum physics).
- Light has a charge, but has no mass, yet it can interact with other objects and add to their relative mass by adding to an objects total energy.
- Light is both electricity and magnetism in constant oscillation.
- Light exists in a state of superposition. It can’t be localized in both time and space.
- Different wave lengths of light can be seen as different colors in the “electromagnetic spectrum,” this spectrum can also carry information as radio waves, or carry information as wifi. Different colors and wave types differ by frequency (waves vibrating more frequently are higher energy).
- Light can cook an egg on the sidewalk by speeding up the molecules in an egg (light is also heat).
- Light reflects and refracts based off the laws of quantum probability.
- Light can be trapped in a crystal, and when anything emits heat or light (like fire), it is emitting photons AKA electromagnetic energy AKA light we call “electromagnetic radiation.”
- Light behaves like a transverse wave (with electric and magnetic waves oscillating in space time at 90 degrees to each other, perpendicular to the wave direction, keeping a straight line trajectory despite their transversing and polarization).
- Light waves crest, cancel other light waves, amplify other light waves, and charged fields can gain or lose energy based on interactions (emitting and absorbing photons).
- Light is subject to what was called “spooky action at a distance,” but is now called quantum entanglement. That is, what happens to one photon can affect another paired, but distant, photon despite being separated in space.
- Light seems to react differently when being observed (for example in the double slit experiment).
- Light can power a car or a factory.
- When things burn they emit light, one can tell their energy content from how they burn, because mass is in ways just a measure of energy content.
- Light will travel in a single direction, at the constant speed of “light speed,” forever, if unimpeded.
- Many photons can exist in the same exact spot creating “wave packets,” or just one photon can exist alone in its lowest energy state (still exhibiting a quantum wave-particle nature). The lowest energy state possible for a photon can be considered a single photon (although one could argue that state can be zero or infinitely small). Meanwhile, there is no limit to how many photons can be in one space (lasers are created from fitting many photons in a small space, this increases the energy content of the wave packet, and can be used to make precession “cuts” via the “heat” created).
Light, it has lots of properties, and is in many respects the basis of our universe. We call it by many names, but it is always the same core thing, “photons,” a charged part of the electromagnetic field.
These simple(-ish) sentences pertaining to the dual wave-particle quantum nature of light have many deep meanings beyond what we could express above, and we’ve only just scratched the surface.
We explain more about light and its mind-bending properties below.
What Is Light? Or, a less complicated question, “what isn’t light?”The Photon, the Carrier Particle of Electromagnetic Energy
- The photon is the carrier particle of all electromagnetic energy, and one of the four fundamental forces of the standard model of particle physics (the quantum physics model that describes how the building blocks of the universe, the elementary particles, interact with each other).
- The photon is massless and is one of only two massless particles (the other being the gluon). These particles have momentum, but no rest-mass (it is worth noting here specifically that photons always travel light speed unimpeded and never truly rest; they are in a sense “pure motion”).
- The term photon has been around for decades, and it stuck due to simplicity. Even though this terminology is correct, photons aren’t “just particles.” They are a measurable excited state, or localized vibration, in the electromagnetic field that travels in a wave-like motion (quantum field theory).
- Each wavelength/particle typically consists of a packet of waves forming an aggregate wavelength, at its core, a single wavelength in a packet can still be described as a photon, but can also described as a one dimensional vibrating string (superstring theory). Newton called a single “light” particle a corpuscle.
Light Speed and the Momentum of a Photon
- The wave-like pattern it travels in aside, the photon only travels in a single direction only. It has “unidirectional” linear momentum.
- The photon travels at a single and constant speed called light speed (in a perfect vacuum). Light speed is one of the universal constants and is one of the only non-relative things in the universe. As Einstein noted, because light speed is constant time and space are relative (but spacetime, a composite measure of space and time, isn’t).[3][4]
- The photon can travel over an infinite distance unless impeded.
- Light doesn’t “slow down” under normal conditions (it doesn’t change linear momentum in free space), but it can change energy content (slowing its frequency of vibration and cooling down, or increasing frequency and heating up), bounce off objects, and be generally manipulated in strange ways within these criteria (see here and here).
- The photon also has spin (angular momentum). It, like other force carrier particles, has a spin of “1”. Matter particles (fermions) have a spin of “+ or – 1/2”.
- Since a photon has a spin of “1” it doesn’t have to adhere to the Pauli Exclusion principle and thus more than one photon can be in the same place and the same time. Lasers are made from condensing many photons into one spot, this increases the energy content of the packet of photons, and results in a really “hot” laser.
FACT: Photons can’t interact with each other directly (outside of forming wave packets and absorbing and emitting other photons) and have no rest-mass when traveling through empty space, yet in special circumstances they can exist as Photonic matter. Photonic matter phenomenon where photons interacting with a gas develop apparent mass, and can interact with each other, even forming photonic “molecules.”
The Speed of Light is NOT About Light | Space Time | PBS Digital Studios.Electromagnetic Radiation and the Electromagnetic Spectrum
- A photon can emit other photons and can absorb other photons (within limits), this changes the photon’s energy content and frequency.
- Other quantum particles can also absorb and emit photons; this is what happens when electrons change energy states.
- Electromagnetic radiation describes emitted photons, like the ones that we call visible light (although not every emitted photon is visible).
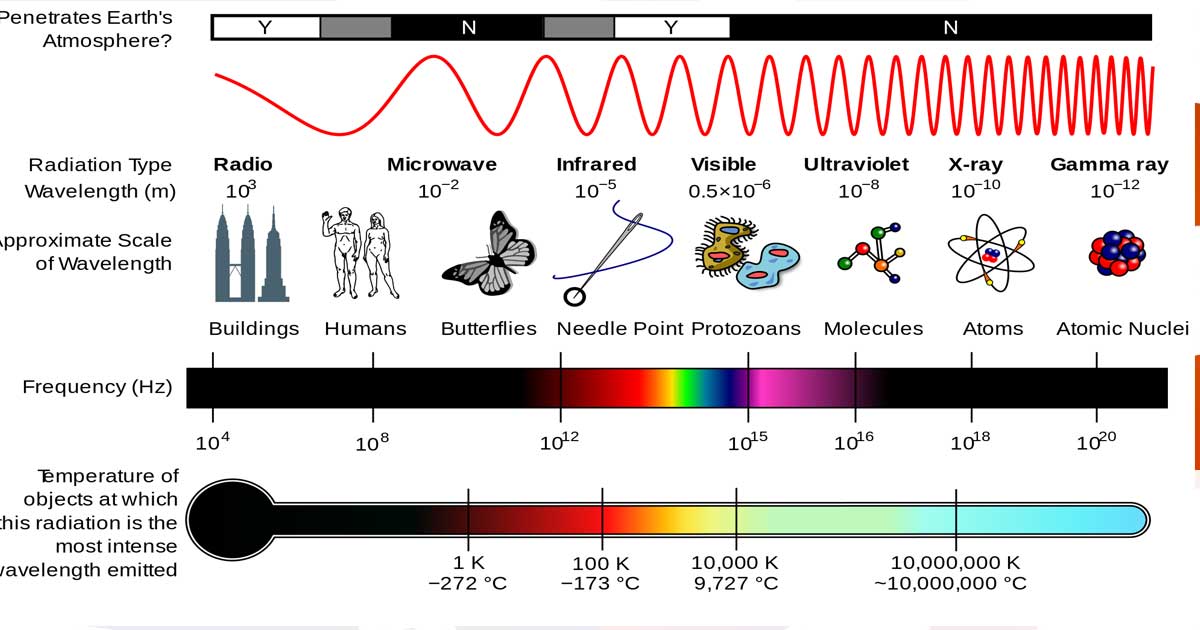
- Visible light is a specific range of frequencies in the electromagnetic spectrum (precise frequencies of electromagnetic radiation that the human eye can see).
- The electromagnetic spectrum describes all the possible frequencies of vibration in the electromagnetic field, the visible spectrum describes the wavelengths we can see with the naked eye.
- Different “size” waves have different properties, and each color of light has a unique range of vibrations.
- White light describes multiple colors of light and can be separated into different colors of light (like Newton did with a prism and wrote about in his book Opticks).
- The energy content of a photon is determined by its frequency times Max Planck’s constant (which represents the universe’s smallest unit). The equation is simply E=hf.
- Despite the Planck representing the smallest unit, the minimum wavelength of a photon is zero and there is no maximum. Despite the quantizing nature of quanta, there is, in one sense, no minimum or maximum energy content of a photon. The rule is that it must be a positive integer greater than zero (it must have a frequency greater than zero).
- Frequency is a way to measure waves and vibrations by examining their patterns. The wavelength is the distance between successive crests of a wave. The higher the frequency, the shorter the distance between crests.
- Only certain frequencies of electromagnetic radiation produce visible wavelengths. Short waves are toward blue tones, including ultraviolet, which bees can see, and X-rays. Long waves are toward red and include infrared, which the pit viper can see, and radio waves.
Light, Both a Particle and a Wave
- The photon’s wave and particle qualities are two observable aspects of a single phenomenon. In 2015 the long-standing theory that light is both a particle and a wave was confirmed and published in Nature Communications.
- The photon doesn’t just travel in “a wave,” it travels as a transverse wave specifically. Transverse describes the direction the wave moves in (wave vibrating at right angles to the direction of its propagation).
- Electromagnetic energy is a quantum wave, and it doesn’t need to travel through a medium. It is not a mechanical wave like gravity or sound (that travels through a medium).
- All waves transfer energy; electromagnetic waves are no different.
- Electromagnetic energy oscillates in a “near field” of electricity and magnetism. The term “far field” describes the potentially infinite field in which the photon travels via linear momentum (forward movement).
The Quantum Nature of Light, Quantum Fields, and the Photon
- As noted above, the photon is a quantum particle or a quanta. “Quantum” describes the fact that photons jump to discrete states in the electromagnetic field. Instead of moving in a continuous wave, it “quantizes” to probable locations.
- All quantum particles, including the photon, exist as localized vibrating states in their respective field and move as quantized transverse waves. This behavior is explained by Quantum Field theory (QFT) and this behavior is where quantum physics gets its name.
- The electromagnetic field is a single field that covers all of space and time, like a container for electromagnetic energy. Each particle of the standard model of particle physics has its own field.
- When the electromagnetic field contains a localized vibration, we call it a photon.
- Each particle has a separate field. Particle interactions occur when charged excited states in fields overlap.
Probability, Uncertainty, and Reflection
- The quantum behavior of light is described by laws of probability, uncertainty (of position and speed simultaneously), and quantum mechanics.
- When photons reflect off a surface, they reflect based on probability. For example, instead of one out of every four photons bouncing off a surface, 25% of all photons that hit a surface bounce off. There is no way to tell what a single photon will do. Reflection is a matter or odds, not a certainty. Most quantum behavior works this way.
- We can’t “perfectly” localize a photon as it has no mass (and only momentum), and thus it can’t exist without moving (one reason why its better to generally describe light as a wave function of probabilities). We can predict a range of places in which a photon will travel, but we can’t determine it with certainty or localize it perfectly. Or rather, the uncertainty principle says “the more precisely the position of some particle is determined, the less precisely its momentum can be known, and vice versa,” this is due to its quantum nature. It only gets more complex when we consider the bizarre effects of the double slit experiment and quantum entanglement.
Heisenberg, Feynman, Maxwell, Planck, and Einstein
- Heisenberg’s uncertainty principle describes the uncertainty of quantum mechanics noted above.
- Maxwell’s equations describe light using mathematics. Einstein showed us that kinetic energy was equivalent to a body’s mass and the constant speed of light. We can use Max Planck’s work to see that kinetic energy is also equal to Planck’s constant multiplied by frequency.
- Later, Richard Feynman broadened the theory of Quantum ElectroDynamics (QED), which describes the nature of light, sometimes using shockingly simple Feynman diagrams.
The Big Picture, Light and the Other Forces
- Electromagnetic energy describes all energy that isn’t dark energy, gravitational energy, or (weak or strong) nuclear force. This includes all kinetic energy such as heat and even the energy stored in calories.
- We can consider most of the energy bound to particles as charge or motion to be photons. When particles interact they exchange virtual bosons (the name for all the carrier particles of the four forces).
- When we think of all four forces together – electromagnetic, gravity, and both nuclear forces, and their respective “bosons” – then we can say energy is simply the kinetic and bound potential energy of a system in any form. We can describe this in one word, mass-energy.[5]
- We can sum this up by saying, “All elementary particles, including light, exhibit properties of mass-energy, and are understood as wave-like quantum fields, that interact with other quantum fields, in excited quantized states called particles.”
- You are starting to understand quantum physics if you can picture the physical universe as composed of massless vibrating energy field interactions in which everything is relative to their nature. Constants derived from the behavior of these fields provide our only reliable measure of what is real.
